The Ozone Hole in Antarctica
The seasonal thinning of the ozone layer of the earth's atmosphere above Antarctica, so allowing abnormal amounts of ultra-violet light to reach the earth's surface in those regions.
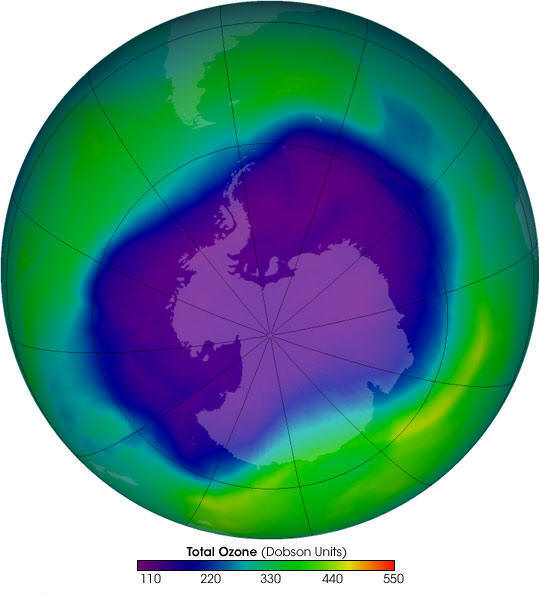 300
units (green) is normal, less than 220 (blue) is a "hole"
300
units (green) is normal, less than 220 (blue) is a "hole"
What is ozone?
Ozone is a gas* made of oxygen atoms. Usually oxygen atoms hang around in pairs - this is the sort of oxygen that we breathe and that helps things to burn. Oxygen sometimes however will form a molecule with three oxygen atoms, this is what we call ozone:O2 - two oxygen atoms - ordinary oxygen
O3 - three oxygen atoms - Ozone
Ozone has a the particularly useful characteristic that it can absorb large quantities of ultra-violet (uv) light - more of that soon.
*Jumping Jack Flash is also a gas.
Where is the ozone?
Most of the ozone on earth isn't on earth at all, but in the layer of the earth's atmosphere called the stratosphere. This is the upper layer of the atmosphere and starts between 12.9 to 19.3 km (8 to 12 miles) above our heads and goes upwards to almost 50 km (30 miles). The stratosphere has virtually no clouds or other form of weather, it's thinnest at the equator and thickest at the poles.Ozone is formed in the stratosphere by the action of sunlight on oxygen molecules. In particular it is the high energy ultra-violet light in sunlight that is effective, it causes an oxygen molecule to split into two oxygen atoms:
O2
--------> O
+ O
oxygen molecule
2 oxygen atoms
These lone oxygen atoms are very reactive, each then joins with another oxygen molecule to form a molecule of ozone:
O
+ O2
--------->
O3
oxygen atom + oxygen molecule
ozone molecule
Ozone may also be destroyed by joining with a lone oxygen atom to get back to oxygen again. Ultra-violet light is required for ozone to form in the stratosphere, but then the ozone absorbs the ultra-violet light so stopping it reaching deeper into the earth's atmosphere. The result is that levels of ozone are greatest at around 20km up. This is good news for us as it stops lots of ultra-violet light getting through to us and also keeps the ozone high up in the atmosphere out of the way.
Isn't ozone bad news? I'm sure I saw something on the weather forecast....
Ironically, at ground level ozone is very bad news. It is a major component of photochemical smog. Down here on the ground, It is caused by the effect of ultra-violet light on nitrogen oxide from vehicle exhausts and so particularly affects built up areas in regions of high sunshine.
Ozone affects lung function, it can aggravate asthma and other chronic respiratory tract and lung diseases and can reduce lung function in the short term or even permanently on repeated exposure. Ozone has an effect like sunburn on the lining of the respiratory tract damaging the cells.
Why is a ozone hole a problem?
Ozone in the stratosphere is nicely out of the way and has the wonderful benefit to life on earth that it specifically absorbs the harmful ultra-violet light from the sun while letting other light wavelengths through.
Though we talk about a "hole"
in the ozone layer, it's not an actual hole, just a
thin bit. Ozone is spread thinly throughout the stratosphere
- and in low quantities too - if all the ozone above
your head was collected together in a continuous layer,
it would only be about 3-5mm (1/8 of an inch) thick.
It's better to think of the ozone
as being like orange squash in a glass of water where the
water is the rest of the atmosphere. When we talk about
an "ozone hole" we actually mean a region where
the squash is thinner and more diluted than we'd like
it to be.
When a "hole" forms in the ozone layer then this means that more harmful ultra-violet rays get through than are good for us or many other life forms, plant or animal as there isn't enough ozone to stop it.
Too much ultra-violet light can result in:
- Eye damage such as cataracts.
- Immune system damage.
- Reduction in phytoplankton in the oceans
that forms the basis of all marine food chains including
those in Antarctica.
- Damage to the DNA in various life-forms
So far this has been as observed in Antarctic ice-fish that
lack pigments to shield them from the ultra-violet light
(they've never needed them before).
- Skin cancer.
- Probably other things too that we don't know about at the moment.
Climate effects
The depletion of the ozone hole has also caused an overall cooling trend on the Antarctic continent, this has masked to some extent the effects of warming temperatures, particularly on the larger part of East Antarctica and areas away from the peninsula region.
The loss of ozone has also led to increased winds and storms, both in frequency and strength. Winds in the Southern Ocean have been estimated to have increased by 15-20%. It has caused a low pressure system to form in the Amundsen Sea again both with increased frequency and strength. This low pressure sucks cold air from the interior of Antarctic and across the Ross Sea leading to a great increase in the amount of sea-ice forming in this area in recent years.
Why does a ozone hole form over Antarctica?
The ozone hole is caused by the effect of pollutants in the atmosphere destroying stratospheric ozone. During the Antarctic winter something special happens to the Antarctic weather.
- Firstly, strong
circular winds form that blow around the whole continent,
this is known as the "polar vortex" - this
isolates the air over Antarctica from the rest of the world.
- Secondly, special clouds form called Polar Stratospheric Clouds. Clouds don't normally form in the stratosphere and these turn out to have the effect of concentrating the pollutants that break down the ozone, so speeding the process up.
 Polar stratospheric clouds at about 25,000 m
(80,000 ft) altitude. These are the highest flying of all
clouds and only occur in polar regions where the temperature
in the upper atmosphere dips below minus 70C (-100F). They
are sometimes called "nacreous clouds" as they
are coloured like the nacre of mother-of-pearl with coloured
bands that move the position of cloud and observer.
Polar stratospheric clouds at about 25,000 m
(80,000 ft) altitude. These are the highest flying of all
clouds and only occur in polar regions where the temperature
in the upper atmosphere dips below minus 70C (-100F). They
are sometimes called "nacreous clouds" as they
are coloured like the nacre of mother-of-pearl with coloured
bands that move the position of cloud and observer.
Picture courtesy Alan Light -
more pictures of Polar Stratospheric Clouds
By the time spring arrives and the sun comes back after the long polar night, the ozone levels are severely depleted around the Antarctic continent causing the "ozone hole". Unfortunately, there then follows a particularly long period of high sunshine and long days, just to make the effect of the ozone hole worse with all that uv light around.
The concentration of ozone in the atmosphere is measured in "Dobson Units", the average concentration of ozone in the atmosphere is about 300 Dobson Units. The ozone hole is considered to be wherever the concentration drops below 220 Dobson Units.
The following pictures show the extent of ozone thinning. Dark blue and purple colors correspond to the thinnest ozone, while light blue, green, and yellow pixels indicate progressively thicker ozone.
October 1999 (average)
Historically, the Antarctic ozone hole was
largest during October. In recent years however, September
has been the peak month.
September 7th 2000
The ozone hole grew quicker than usual and
exceptionally large. By the first week in September
the hole was the largest ever at that time. For the
first time it reached towards South America and to regions
of high population.
September 3rd 2020
In September 2020, the average area of the ozone hole
was the largest ever seen, at 28.3 million square kilometres
(11 million square miles). The previous record was about
27.2 million square kilometres (5 million square miles)
on September 19th, 1998.
The ozone hole builds up over the winter months, peaking at around September and breaking up again by December, this data set from 2020.
July
August
September
October
November
December
What causes the ozone depletion?
 Ozone
is mainly broken down by chemicals called ChloroFluoroCarbons
CFC's and also by nitrogen oxides.
Ozone
is mainly broken down by chemicals called ChloroFluoroCarbons
CFC's and also by nitrogen oxides.CFC's ironically were first used in large quantities because they were thought to be safe and inert (unreactive) chemicals. They are a group of chemically similar gases used in refrigeration systems, air conditioners, aerosols, solvents and in the production of some types of packaging. Nitrogen oxides are a by-product of fuel burning, they find their way to altitude where they can damage ozone come from jet aircraft exhausts.
CFC's don't occur naturally, they are man-made chemicals. They are very useful when they are where they are supposed to be, and doing what they are supposed to be doing. But once released into the atmosphere they are a serious pollutant. The problem was it took us many years to realise this during which time we thought they were perfectly harmless, but in fact were building up to levels that will take decades to disappear again even when we stopped producing them altogether.
The reactions that destroy ozone are very complicated. They take place on the surface of the ice particles of the Polar Stratospheric Clouds and it takes only a small amount of CFC to destroy an awful lot of ozone.
Is the ozone hole going to stay over Antarctica?
Since the annual thinning of the ozone layer over Antarctica was first discovered, measurements have been carried out in all regions. Ozone depletion has been measured everywhere in the world except in the tropics. Depletion is usually worse the further from the equator and recently an Ozone hole (as defined by a distinct area of very low ozone levels) has been detected above the North Pole in the arctic.
There is a lot to learn about the breakdown of ozone in the atmosphere and warmer regions, non polar depletion of ozone in particular is not properly understood.
So for the time being the "ozone hole" seems to be an Antarctic phenomena, but a less severe thinning of the ozone layer is pretty much a world-wide thing. How acute and important it will be in the future is not known.
Can the ozone hole recover?
Yes! - hurrah!
The way to stop the formation, growth and spread of ozone thinning is to reduce the production of those chemicals that cause the destruction of ozone, namely CFC's and nitrogen oxides.
In 1987, the Montreal Protocol was signed by many nations whereby those nations that signed agreed to reduce their emissions of CFC's to a half (of the 1987 levels) by 2000.
Potential problems come from nations that do not see the reduction of CFC's to be a priority, and also from the huge quantity of refrigeration and air conditioning systems in the world that still contain CFC's. If they are not disposed of correctly, then the CFC's will escape into the atmosphere and continue to destroy ozone.
The latest estimates are that as long as production and release of CFC's is regulated properly, global ozone levels should recover by 2050, this may seem a long way off, though the indications are that we are on target for it, the Montreal Protocol was 36 years ago, 2050 is 27 years away (I'm writing this in 2023), so it is doable. We just have to make sure we don't start to use CFC's again.
Picture credits, copyright pictures used by permission: All ozone hole images - NASA - Ozone hole watch
